Antrodia cinnamomea alleviates cisplatin-induced hepatotoxicity and enhances chemo-sensitivity of line-1 lung carcinoma xenografted in BALB/cByJ mice
Abstract
Whereas cisplatin (cis-diamminedichloroplatinum II) is a first-line medicine to treat solid cancerous tumors, it often causes serious side effects. New medicines that have an equivalent or even better therapeutic effect but with free or less side effects than cisplatin are highly anticipated in cancer therapy. Recent reports revealed that Antrodia cinnamomea (AC) possesses hepatoprotective activity in addition to anticancer. In this study, we wanted to know whether AC enhances chemo-sensitivity of cisplatin and/or alleviates cisplatin-induced hepatotoxicity, as well as the underlying mechanisms thereof. Our results indicated that AC inhibited proliferation of line-1 lung carcinoma cells and rescued hepatic HepG2 cells from cisplatin-induced cell death in vitro. The fact is that AC and cisplatin synergized to constrain growth of line-1 lung carcinoma cells in BALB/cByJ mice. Quantitative real-time PCR further revealed that AC promoted expression of apoptosis-related genes, while it decreased expression of NF-κB and VEGF in tumor tissues. In liver, AC reduced cisplatin-induced liver dysfunctions, liver inflammation and hepatic apoptosis in addition to body weight restoration. In summary, AC is able to increase cisplatin efficacy by triggering expression of apoptosis-related genes in line-1 lung cancer cells as well as to protect liver from tissue damage by avoiding cisplatin-induced hepatic inflammation and cell death.
Figures 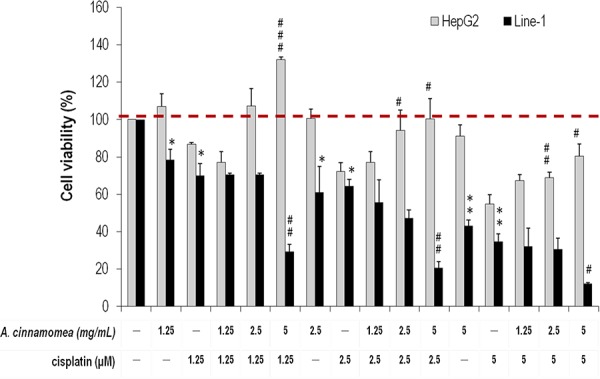
Figure 1. Effects of A. cinnamomea on…
Figure 1. Effects of A. cinnamomea on cell viability in cisplatin-treated human hepatic HepG2 and…
Figure 1. Effects of A. cinnamomea on cell viability in cisplatin-treated human hepatic HepG2 and mouse line-1 lung carcinoma cells Cells were incubated in culture medium containing various concentrations of cisplatin and/or A. cinnamomea for 48 h. After the treatment, cell viability was determined by the MTS assay. Values shown are relative to that of vehicle control, where the value of control cell viability is set to 100%, a representative of three independent experiments. Data, which are derived from at least three independent experiments, six tests for each, are presented by mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle-treated cells; #P < 0.05, ##P < 0.01 versus cisplatin-treated cells.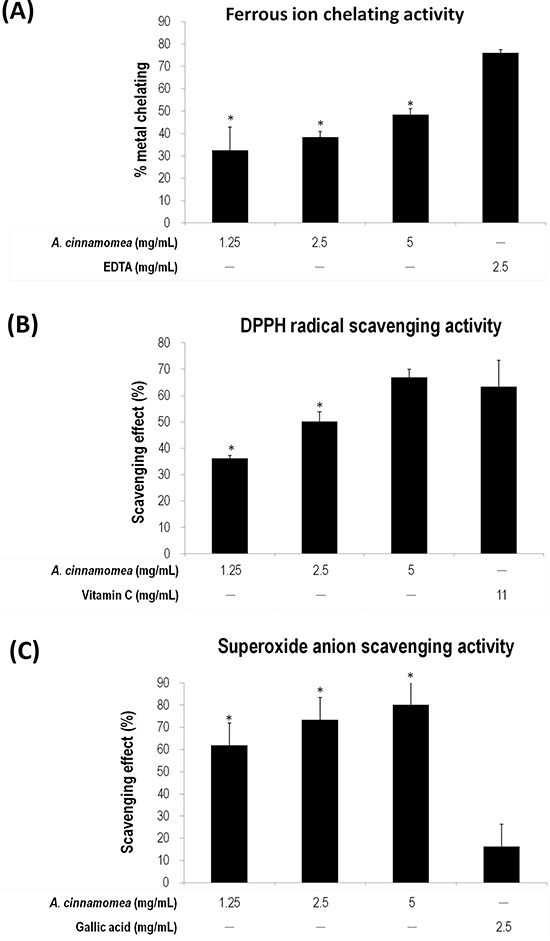
Figure 2. Antioxidation activity of A. cinnamomea
Figure 2. Antioxidation activity of A. cinnamomea
The antioxidation activity for A. cinnamomea was determined…
Figure 2. Antioxidation activity of A. cinnamomea The antioxidation activity for A. cinnamomea was determined by the ferrous ion chelating assay. A. the α,α-diphenil-β-picrylhydrazine (DPPH) radical scavenging assay. B. and the superoxide anion scavenging assay C. Data, which are derived from at least three independent experiments, six tests for each, are presented by mean ± SEM. *P < 0.01 indicates significant difference from the positive control.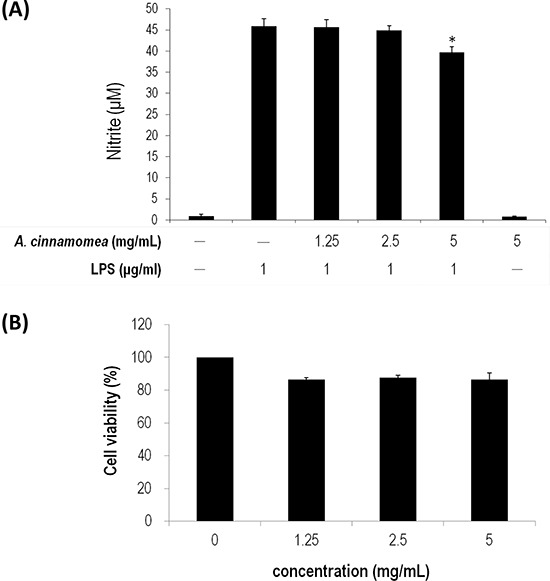
Figure 3. Inhibition of A. cinnamomea on…
Figure 3. Inhibition of A. cinnamomea on NO production in LPS-stimulated RAW264.7 macrophages
A. Various… Figure 3. Inhibition of A. cinnamomea on NO production in LPS-stimulated RAW264.7 macrophages A. Various concentrations (1.25, 2.5 and 5 mg/mL) of A. cinnamomea were evaluated for NO production in LPS-stimulated RAW264.7 macrophages. B. Cytotoxicity of A. cinnamomea was determined by the MTS assay. Values shown are relative to vehicle control, where the value of control cell viability is set to 100%, a representative of three independent experiments. Data, which are derived from at least three independent experiments, six tests for each, are presented by mean ± SEM. 1 μg/mL LPS is a positive control. *P < 0.01 indicates significant difference from the positive control.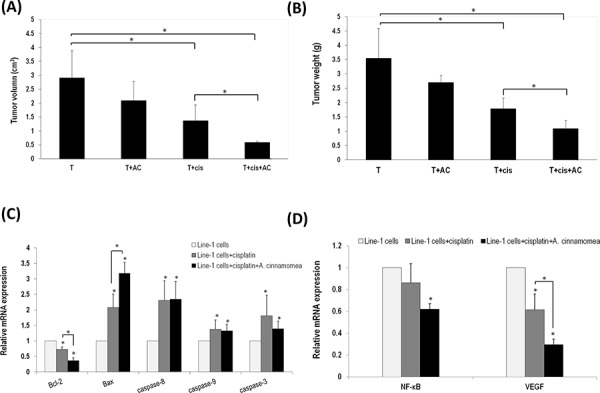
Figure 4. A. cinnamomea and cisplatin synergistically…
Figure 4. A. cinnamomea and cisplatin synergistically inhibit tumor growth in the line-1 xenografted mouse…
Figure 4. A. cinnamomea and cisplatin synergistically inhibit tumor growth in the line-1 xenografted mouse model A. Line-1 lung carcinoma cells were established in BALB/cByJ male mice (n = 6) by subcutaneously injecting line-1 cells (3 × 105 cells) into the right thigh. Mice were orally administered with vehicle (T), 300 mg/kg A. cinnamomea (T+AC), 2.5 mg/kg cisplatin (T+cis), or a combination of A. cinnamomea and cisplatin (T+cis+AC) for 18 days. Mice were sacrificed on day 26 and then examined for their tumor volumes. Tumor volumes were calculated using the following formula: (length × width2)/2. B. Examination of tumor weights. C. Estimation of mRNA levels of Bacl-2, Bax, caspases 3, 8 and 9 by quantitative real-time PCR (qRT–PCR) from tumors treated with vehicle control, cisplatin, or the combination of A. cinnamomea and cisplatin. D. Quantification of mRNA levels of VEGF and NF-κB by qRT–PCR in the tumor tissues of all mice treated with vehicle control, cisplatin, or the combination of A. cinnamomea and cisplatin. The value of the vehicle control mRNA is set to 1. Data are expressed as the means ± SD (n = 6 mice per group from two independent experiments). *Significantly different from the compared group (P < 0.05).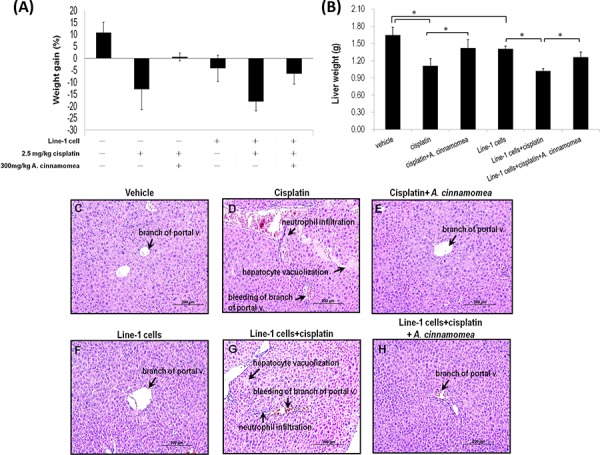
Figure 5. Effects of A. cinnamomea on…
Figure 5. Effects of A. cinnamomea on cisplatin-induced body weight and liver weight loss in…
Figure 5. Effects of A. cinnamomea on cisplatin-induced body weight and liver weight loss in the line-1 xenografted mouse model At day 26, mice were sacrificed and examined for the final gains of body weights A. and livers weights B. Data are expressed as the means ± SD (n = 6 mice per group from two independent experiments). *Significantly different from the compared group (P < 0.05). C–H. Histopathological observation of liver. (C) vehicle group; (D) 2.5 mg/kg cisplatin treated group (cis group) showing severe bleeding, markedly inflammatory cell infiltration and vacuolar degeneration in some hepatocytes; (E) 2.5 mg/kg cisplatin +300 mg/kg AC treated group (cis+AC group) showing no pathological changes except for a few bleeding in the sinusoids; (F) line-1 cell- inoculated group (T group); (G) line-1 cell-inoculated group treated with 2.5 mg/kg cisplatin (T+cis group) showing severe bleeding, markedly inflammatory cell infiltration and vacuolar degeneration in some hepatocytes; (H) line-1 cell-inoculated group treated with 2.5 mg/kg cisplatin plus 300 mg/kg AC (T+cis+AC) showing no pathological changes except for a few bleeding in the branch of portal vessles. Results from 6 representative animals are shown. (magnification, all 200×).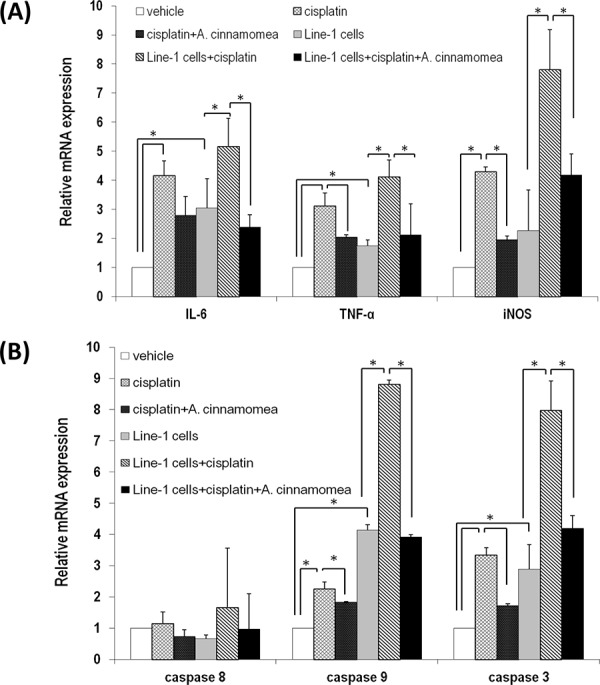
Figure 6. A. cinnamomea decreases cisplatin-induced inflammation…
Figure 6. A. cinnamomea decreases cisplatin-induced inflammation and cell apoptosis in liver
A. Quantification of… Figure 6. A. cinnamomea decreases cisplatin-induced inflammation and cell apoptosis in liver A. Quantification of mRNA levels of inflammatory cytokines by qRT–PCR in the liver tissues of all mice treated with vehicle control, cisplatin, or the combination of A. cinnamomea and cisplatin. B. Quantification of mRNA levels of caspases 3, 8 and 9 by qRT–PCR in the liver tissues of all mice treated with vehicle control, cisplatin, or the combination of A. cinnamomea and cisplatin. The value of the vehicle control mRNA is set to 1. Data are expressed as the means ± SD (n = 6 mice per group from two independent experiments). *Significantly different from the compared group (P < 0.05).