Anti-Oxidant, Anti-Mutagenic Activity and Safety Evaluation of Antrocin
Abstract
Antrocin is a novel compound isolated from Antrodia cinnamomea, and is classified as a sesquiterpene lactone. The therapeutic efficacy of antrocin has been studied, and it has shown an antiproliferative effect on various cancers. The aim of this study was to evaluate the anti-oxidant activity, potential genotoxicity, and oral toxicity of antrocin. Ames tests with five different strains of Salmonella typhimurium, chromosomal aberration tests in CHO-K1 cells, and micronucleus tests in ICR mice were conducted. The results of anti-oxidant capacity assays showed that antrocin has great anti-oxidant activity and is a moderately strong antimutagenic agent. In the results of the genotoxicity assays, antrocin did not show any mutagenic potential. In the 28-day oral toxicity test, Sprague Dawley rats were gavaged with 7.5 or 37.5 mg/kg of antrocin for 28 consecutive days. In addition, 7.5 mg/kg sorafenib, an anti-cancer drug, was used as a positive control for toxicity comparison. At the end of the study, antrocin did not produce any toxic effects according to hematology, serum chemistry, urine analysis, or histopathological examinations. According to the results of the genotoxicity and 28-day oral toxicity study, antrocin, at a dose of 37.5 mg/kg, did not cause adverse effects and can be a reference dose for therapeutic agents in humans.
Figures 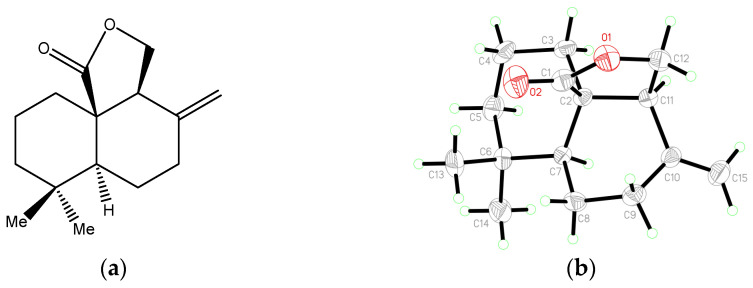
Figure 1
The chemical structure of (-)-Antrocin,…
Figure 1
The chemical structure of (-)-Antrocin, ( a ): chemical formula of antrocin (…
Figure 1 The chemical structure of (-)-Antrocin, (a): chemical formula of antrocin (b): the Oak Ridge Thermal Ellipsoid Plot (ORTEP) diagram of (-)-Antrocin.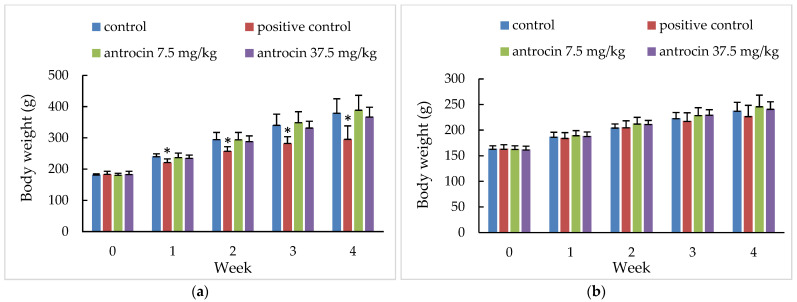
Figure 2
Body weight changes of rats…
Figure 2
Body weight changes of rats treated with antrocin in the 28-day oral toxicity…
Figure 2 Body weight changes of rats treated with antrocin in the 28-day oral toxicity study. Body weight changes are listed in male (a) and female (b) rats. Control: Olive oil, positive control: sorafenib 7.5 mg/kg, antrocin 7.5 mg/kg bw, and antrocin 37.5 mg/kg bw. * Significant difference between the control and treated groups at p < 0.05.