Anti-cachectic effect of Antrodia cinnamomea extract in lung tumor-bearing mice under chemotherapy
Abstract
Skeletal muscle atrophy, the most characteristic feature of cancer cachexia, often occurs in patients with cancer undergoing chemotherapy. Antrodia cinnamomea (AC) a widely used edible medical fungus, exhibits hepatoprotective, anti-inflammatory and anticancer activities. In this study, we investigated whether combined treatment with the ethonolic extract of AC ameliorates cachexia symptoms, especially muscle wasting, in lung tumor-bearing mice treated with chemotherapy. Our results revealed that gemcitabine and cisplatin-induced severe body weight loss and skeletal muscle atrophy in the mice with cancer were greatly attenuated after AC extract administration. The protection may be attributed to the inhibition of skeletal muscle proteolysis by suppressing myostatin and activin release, muscle wasting-related FoxO3/MuRF-1/MAFbx signaling, proteasomal enzyme activity, and pro-inflammatory cytokine production. A significant decrease in insulin-like growth factor 1 (IGF-1) expression and formation was observed in the atrophying muscle of the conventional chemotherapy treatment group (CGC), and this decrease was markedly reversed by AC treatment. Additionally, the anorexia, intestinal injury and dysfunction that occurred in the CGC group were mitigated by AC extract. Taken together, these results demonstrated that the AC extract has a protective effect against chemotherapy-induced muscle atrophy mainly by attenuating muscle proteolysis, pro-inflammatory cytokine production, and anorexia, and activating IGF-1-dependent protein synthesis.
Figures 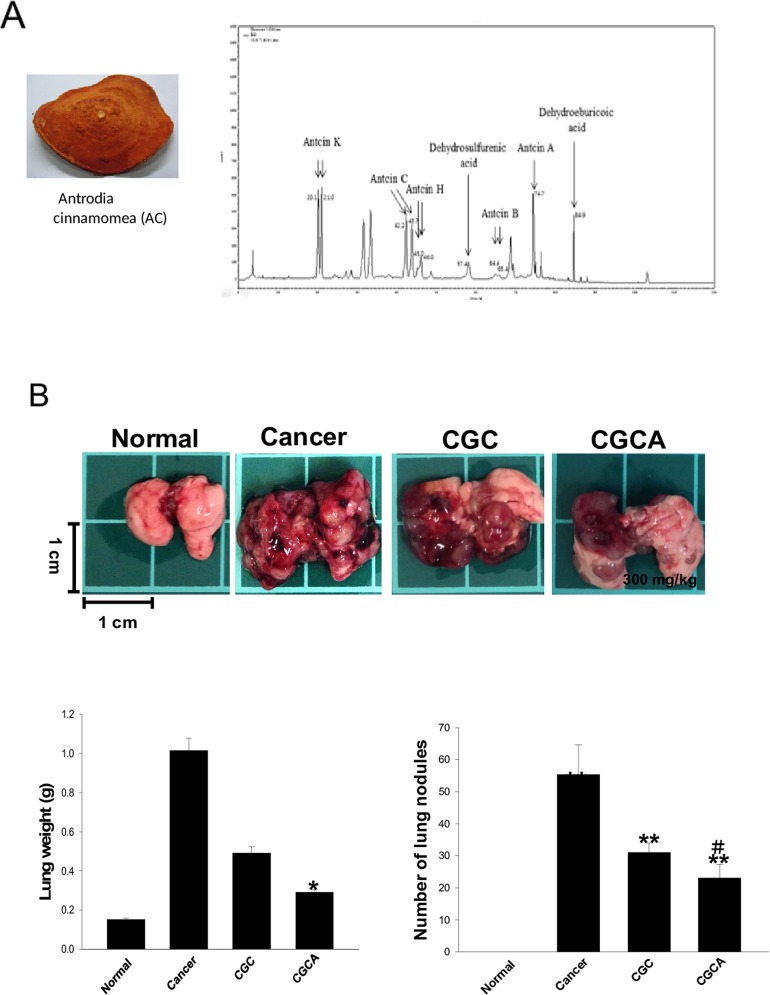
Figure 1. The chemical components of the…
Figure 1. The chemical components of the AC extract and its effects on lung tumor…
Figure 1. The chemical components of the AC extract and its effects on lung tumor growth The morphology of the fruiting bodies of Antrodia cinnamomea (AC) and the representative HPLC profile of the ethanolic extract of AC (A). The images of tumors, the weight, and the tumor nodules of lungs were measured in various groups (B). Data was expressed as mean ± S.E.M. (n=5). *P < 0.05, **P < 0.01, ***P < 0.001 versus the normal group. #P < 0.05 versus the CGC group.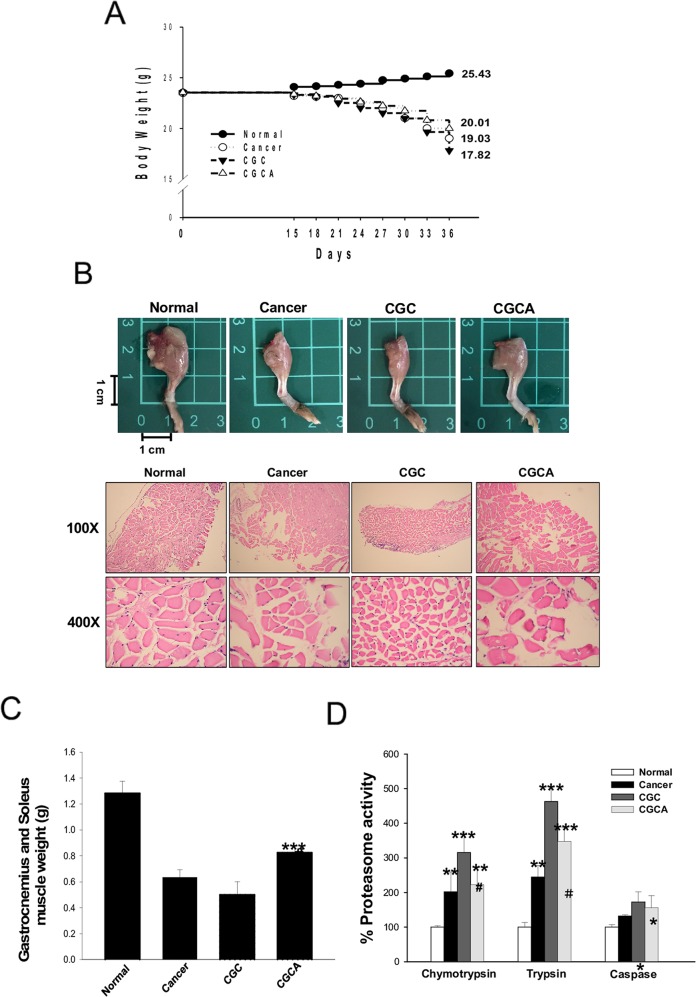
Figure 2. AC treatment attenuates body weight…
Figure 2. AC treatment attenuates body weight loss and muscle atrophy
The body weight (A)…
Figure 2. AC treatment attenuates body weight loss and muscle atrophy The body weight (A), a representative image of the muscle of limb (B), the weight of gastrocnemius and soleus muscle (C), and the proteasome activity (D) were photographed or measured in various groups. Data was expressed as mean ± S.E.M. (n=5). *P < 0.05, **P < 0.01, ***P < 0.001 versus the normal group.#P < 0.05, ##P < 0.01 versus the CGC group.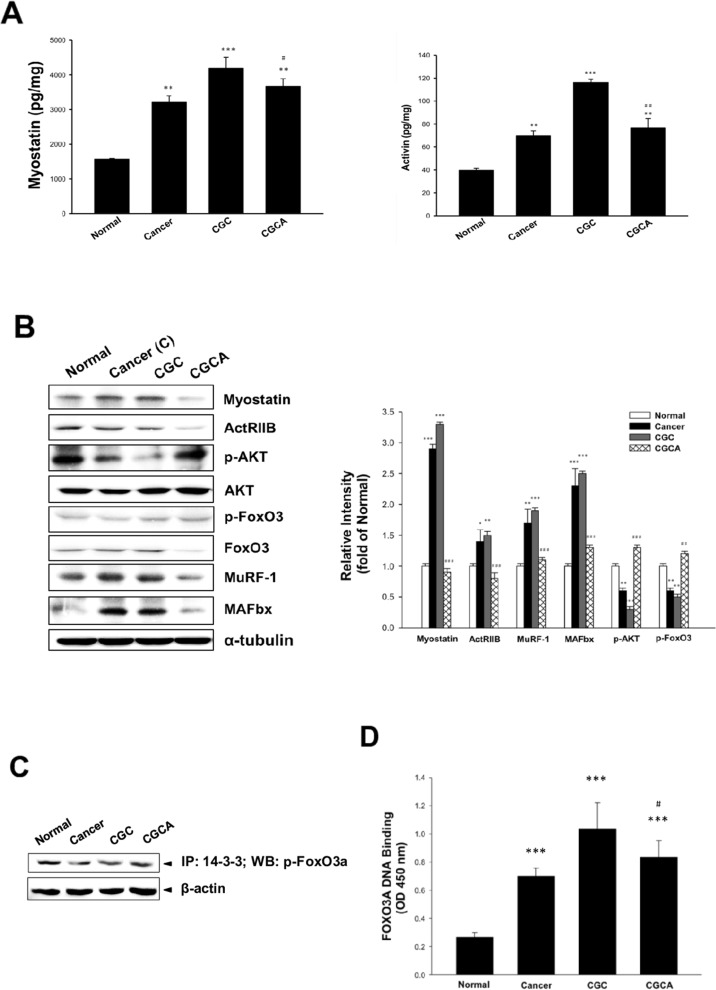
Figure 3. Effects of AC on muscle…
Figure 3. Effects of AC on muscle atrophy-related mediator formation and gene expression
The formation…
Figure 3. Effects of AC on muscle atrophy-related mediator formation and gene expression The formation of myostatin and activin A in muscle (A) and various atrogenic gene expression (B) were determined. The association of p-FoxO3a with 14-3-3 chaperone protein (C) and FoxO3a transcription factor activity (D) in muscle were examined. Data was expressed as mean ±S.E.M. (n=5). *P < 0.05, **P < 0.01, ***P < 0.001 versus the normal group. #P < 0.05, ##P < 0.01 versus the CGC group.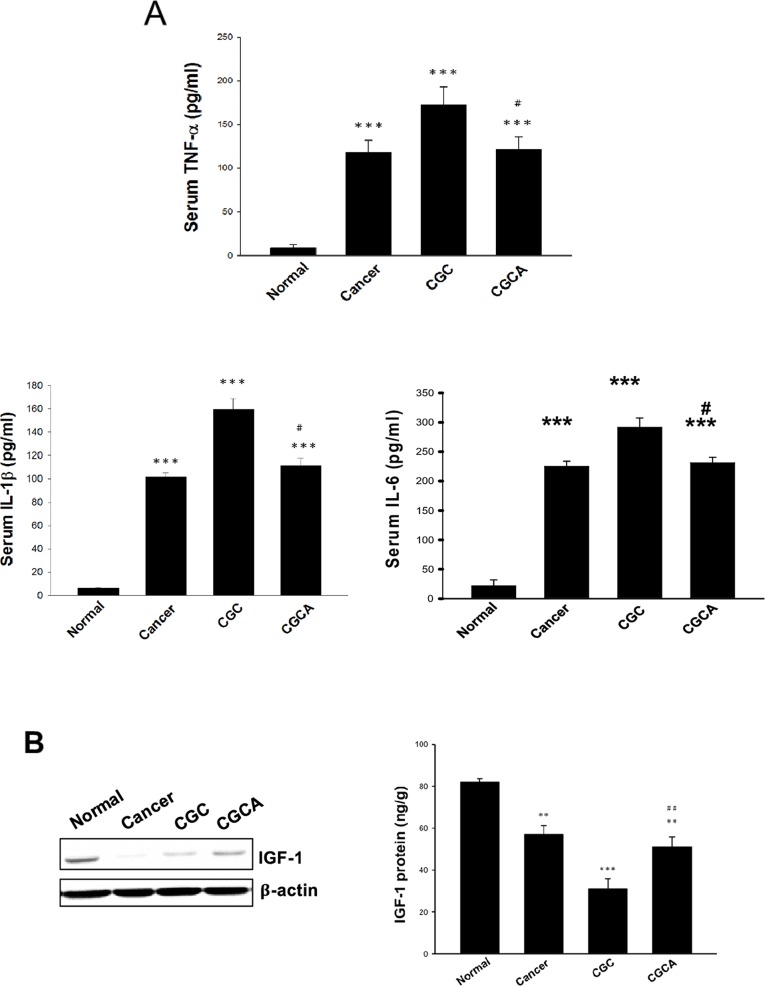
Figure 4. Effects of AC on serum…
Figure 4. Effects of AC on serum pro-inflammatory cytokine levels and IGF-1 expression
The serum…
Figure 4. Effects of AC on serum pro-inflammatory cytokine levels and IGF-1 expression The serum levels of pro-inflammatory cytokines (A), and the expression and amount of IGF-1 in muscle of different groups were measured (B). Data was expressed as mean ±S.E.M. (n=5). **P < 0.01, ***P < 0.001 versus the normal group. #P < 0.05, ##P < 0.01 versus the CGC group.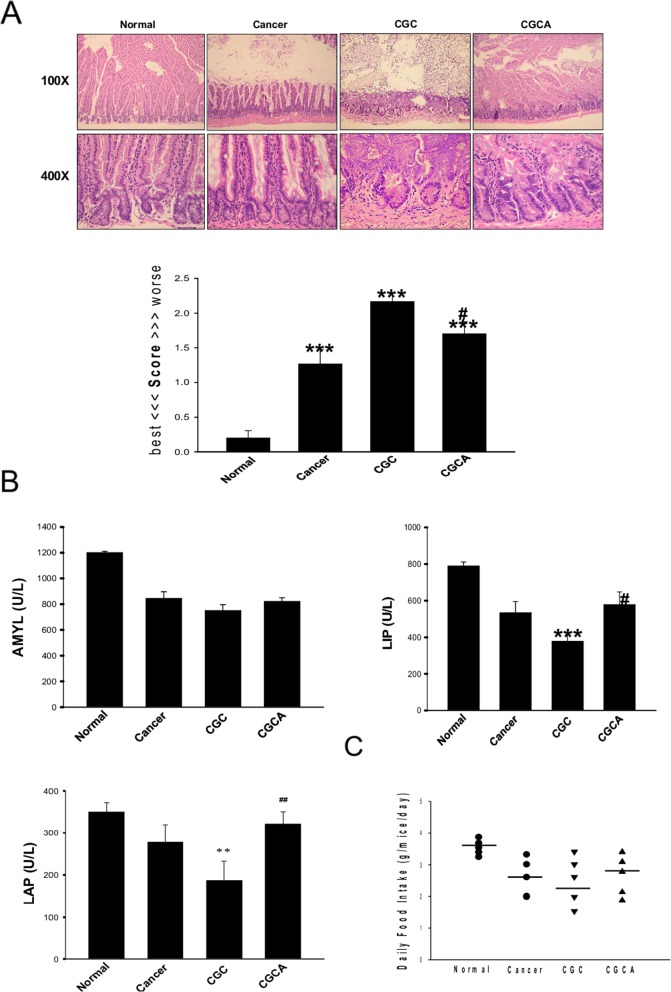
Figure 5. Treatment with AC ameliorates intestinal…
Figure 5. Treatment with AC ameliorates intestinal damage and digestive enzyme dysfunction
The morphological changes…
Figure 5. Treatment with AC ameliorates intestinal damage and digestive enzyme dysfunction The morphological changes and the grading score of the damage in intestines of different groups were evaluated (A). Various intestinal digestive enzyme activity (B) and the daily food intake were measured (C). Data was expressed as mean ±S.E.M. (n=5). **P < 0.01, ***P < 0.001 versus the normal group. #P < 0.05, ##P < 0.01 versus the CGC group.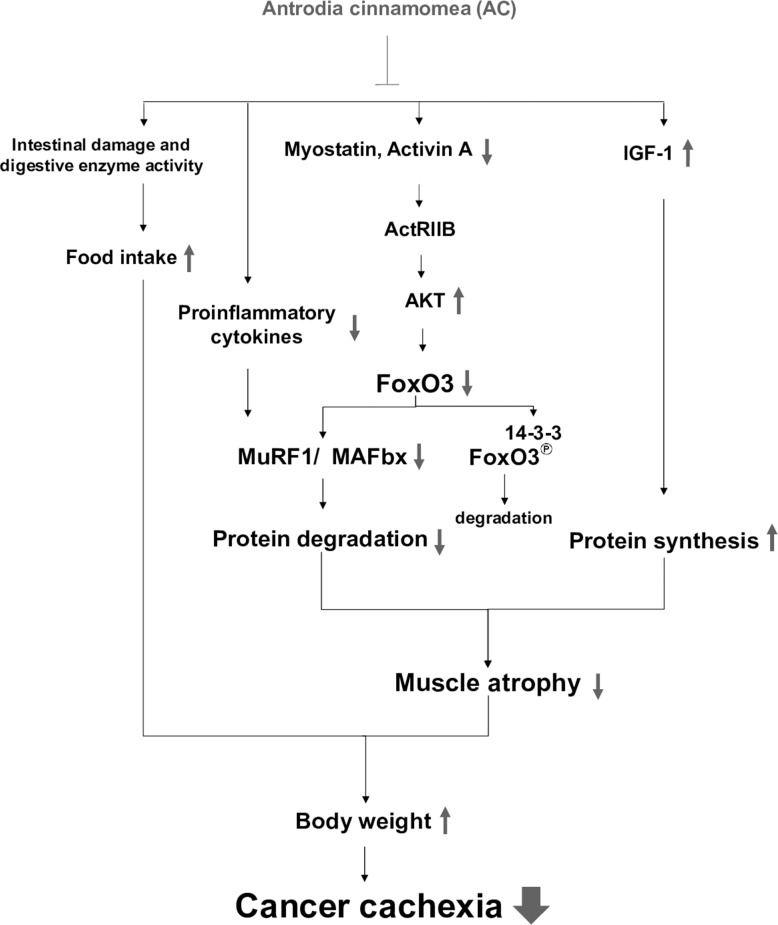
Figure 6. The proposed mechanisms accounting for…
Figure 6. The proposed mechanisms accounting for the anti-cachectic activity of the AC extract
Combined…
Figure 6. The proposed mechanisms accounting for the anti-cachectic activity of the AC extract Combined treatment with AC inhibits myostatin/activin/FoxO3 signaling and pro-inflammatory cytokine formation, leading to the suppression of MAFbx and MuRF1 expression and proteasome activity in muscle, which in turn attenuates muscle proteolysis. Meanwhile, the AC extract enhances IGF-1 expression and its regulated protein synthesis, and improves anorexia and intestinal damage and dysfunction. Therefore, AC has potential to ameliorate cachectic symptoms under chemotherapy, especially body weight loss.