Structure and Anti-Inflammatory Activity Relationship of Ergostanes and Lanostanes in Antrodia cinnamomea
Abstract
Antrodia cinnamomea is a precious edible mushroom originating from Taiwan that has been popularly used for adjuvant hepatoprotection and anti-inflammation; however, the chemical principle for its anti-inflammatory activity has not been elucidated, which prevents the quality control of related products. Using the RAW264.7 model for the anti-inflammatory activity assay as a guide, we reported the isolation and structural elucidation of three potent anti-inflammatory compounds from isolated ergostanes (16) and lanostanes (6). Their structures were elucidated on the basis of spectroscopic data analysis including NMR and HR-QTOF-MS. Particularly, the absolute configurations of (25R)-antcin K, (25R)-antcin A, versisponic acid D, and (25R)-antcin C were determined by single crystal X-ray diffraction (XRD). The representative and most promising compound antcin A was shown to suppress pro-inflammatory biomolecule release via the down-regulation of iNOS and COX-2 expression through the NF-κB pathway while the mRNA levels of IL-1β, TNF-α and IL-6 were also decreased. The high dependency on structural variation and activity suggests that there might be special biological targets for antcin A. Our work makes it possible to develop evidence-based dietary supplements from Antrodia cinnamomea based on anti-inflammatory constituents.
Figures 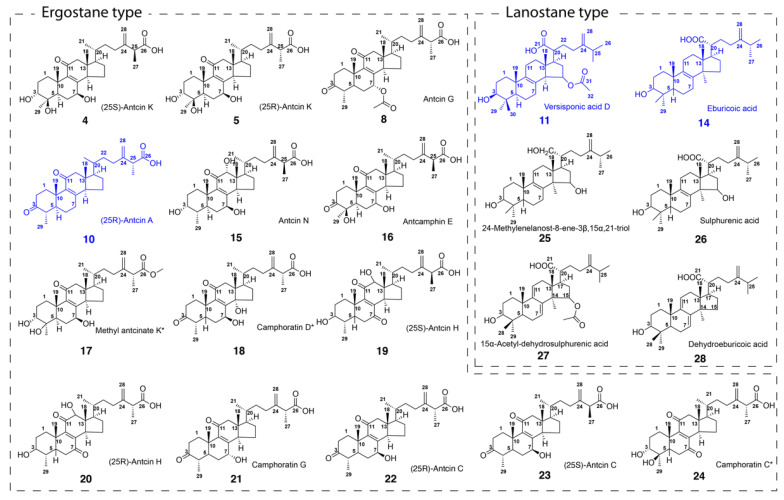
Figure 1
Structures of phytochemicals isolated from…
Figure 1
Structures of phytochemicals isolated from cultured Antrodia cinnamomea fruiting bodies. The blue ones…
Figure 1 Structures of phytochemicals isolated from cultured Antrodia cinnamomea fruiting bodies. The blue ones are potent anti-inflammatory agents. Note: the numbers correspond to the peak numbers in the HPLC chromatogram shown in Figure 2.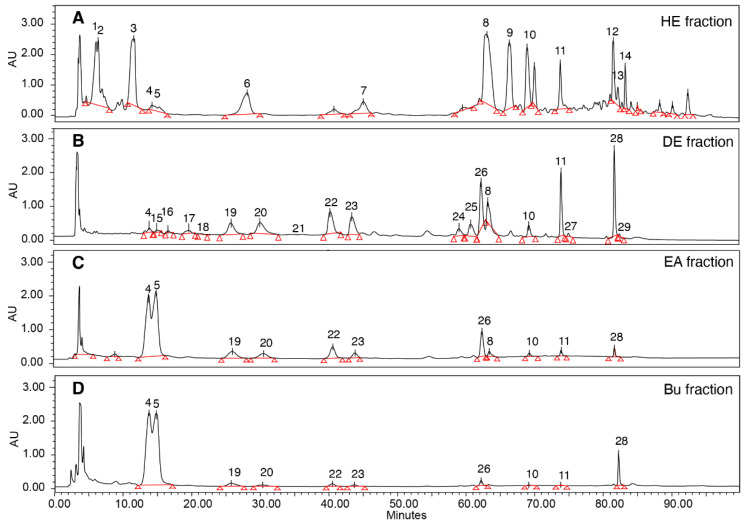
Figure 2
HPLC profiling and bioactivities of…
Figure 2
HPLC profiling and bioactivities of Antrodia cinnamomea fruiting bodies from different fractions. (…
Figure 2 HPLC profiling and bioactivities of Antrodia cinnamomea fruiting bodies from different fractions. (A) Hexane; (B) diethyl ether; (C) ethyl acetate; and (D) n-butanol fractions. (HE: hexane; DE: diethyl ether; EA: ethyl acetate; Bu: n-BuOH).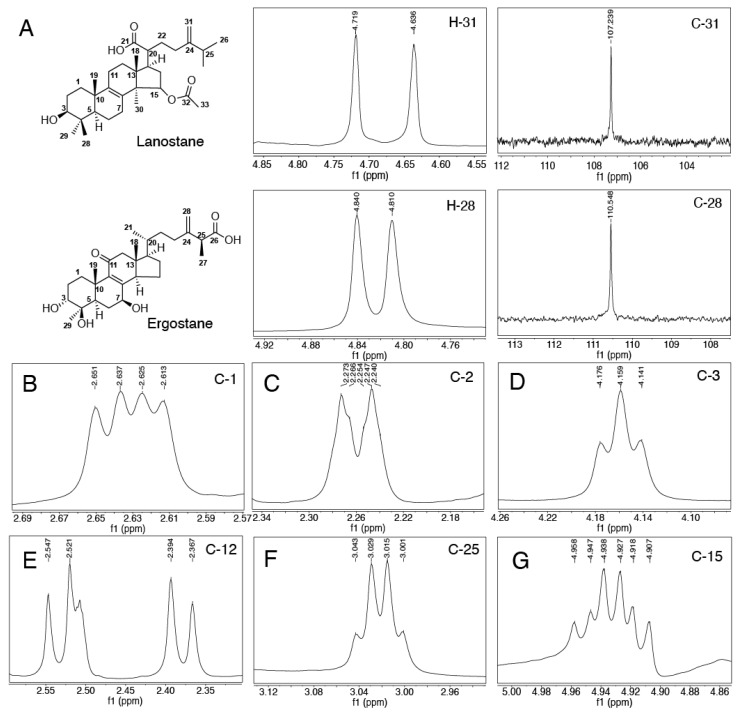
Figure 3
Structural characterization of ergostane-type and…
Figure 3
Structural characterization of ergostane-type and lanostane-type triterpenoids. ( A ) 1 H NMR…
Figure 3 Structural characterization of ergostane-type and lanostane-type triterpenoids. (A) 1H NMR resonance for H−3 and 13C NMR resonance for C-3. The characteristic NMR signal of C1 of (25S)-Antcin K is shown in (B), C2, C3, C12, C25 and C15 in (C), (D), (E), (F) and (G).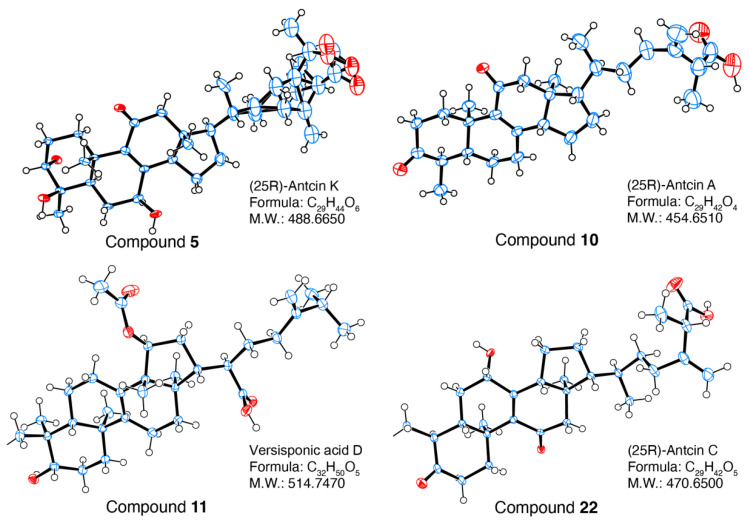
Figure 4
ORTFP drawing of 5 ,…
Figure 4
ORTFP drawing of 5 , 10 , 11 and 22 .
Figure 4 ORTFP drawing of 5, 10, 11 and 22.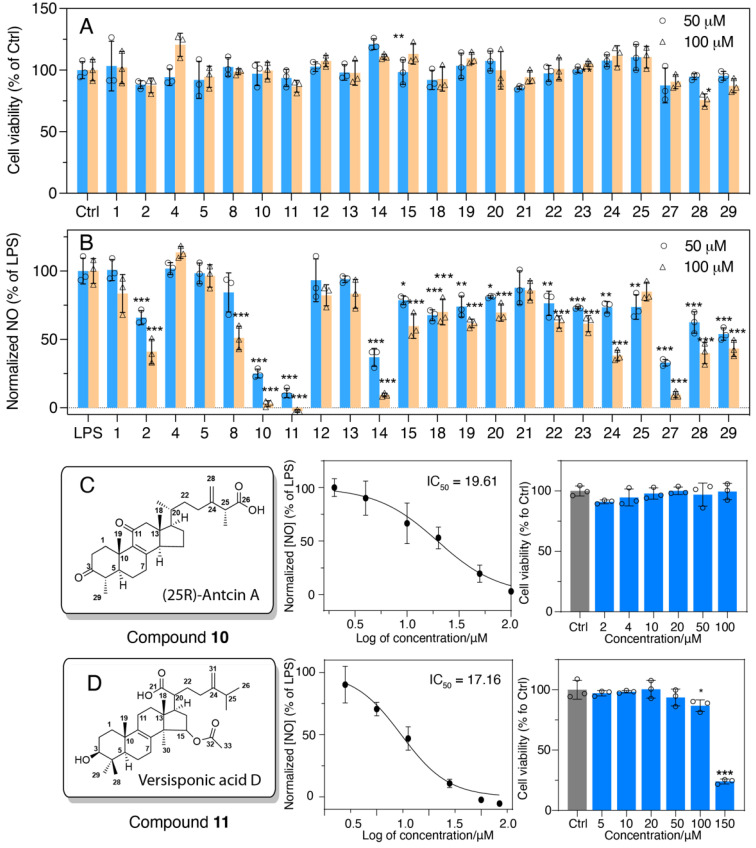
Figure 5
Anti-inflammatory activities of bioactive compounds…
Figure 5
Anti-inflammatory activities of bioactive compounds isolated from Antrodia cinnamomea . ( A )…
Figure 5 Anti-inflammatory activities of bioactive compounds isolated from Antrodia cinnamomea. (A) Cell viability of cells treated only with compounds 1-29. Dose–response curve of normalized NO with the treatment of 100 ng/mL LPS with 10. (B) Nitric oxide production of RAW 264.7 cells treated with 100 ng/mL LPS and compounds 1-29 isolated from Antrodia cinnamomea. (C) Compound 10 and (D) 11 and their cytotoxicity. Results are shown as mean ± S.D, n = 3, p* < 0.05, p** < 0.01, p*** < 0.001 vs. LPS or control.